Trace Elements, Minerals and Vitamins
Cattle and sheep need minerals and vitamins for optimum health and to maximise productivity. The major elements such as calcium and phosphorous are required in relatively large amounts (grams per day) and are known as macro minerals. Minerals required in smaller quantities (milligrammes per day) are known as trace elements or trace minerals. Despite being required in smaller quantities they are no less important than other minerals for optimum animal health and productivity.
Trace elements have four major functions in the body:
- Structural – Minerals and trace elements form structural components of an animal’s body, organs and tissues.
- Catalytic – Minerals and trace elements serve as crucial components of an animal’s enzyme and hormone systems.
- Regulatory – Minerals and trace elements help an animal’s body to regulate key processes such as cell replication, which is an important component of growth.
- Physiological – Minerals and trace elements maintain osmotic pressure, pH balance and are involved in transmission of nerve impulses.
Clinical deficiencies present with extreme clinical signs that are specific to the mineral and the animal. In modern production systems clinical deficiencies are rare but sub-clinical imbalances are much more common. Sub-clinical trace element deficiency is a major cause of loss of productivity in ruminants. Signs of sub-clinical imbalances are often subtle, and onset is gradual, but overtime has a major negative impact on productivity of the animal. Common symptoms include poor growth rates, weak lambs and calves, non-specific ill-thrift or reduced feed intake. An animal’s diet often does not provide adequate levels of trace elements, particularly at certain critical periods of the production cycle; in specific geographical areas where there are chronically low levels of trace elements in the soils and grasses; or where there are high levels of antagonists present which interfere with the utilisation of trace elements. Ensuring an adequate, consistent and reliable supply of trace elements, by appropriate trace element supplementation, supports the animal in meeting its productive potential, and forms part of an integrated health plan, that includes vaccinations and other interventions.
Agrimin’s range of boluses contain a variety of trace elements and vitamins vital to the productivity of your animals. They can help to supplement the trace elements available to them from their diet, thus providing an adequate and consistent supply across various production systems, diets and management practices.

Cobalt
Cobalt is an essential component of vitamin B12, which is essential for the breaking down of feed and a more efficient conversion to liveweight. Vitamin B12 is important to the functioning of two key enzymes. The first enzyme is important in the production of simple sugars (glucose) from components provided via the diet. The second is involved in the production of specific amino acids used to build proteins and other critical molecules. Ruminants cannot synthesise vitamin B12 themselves, it is synthesised by rumen micro-organisms from inorganic sources of cobalt in the diet, thus optimum cobalt in the diet is critical.
Due to its role in the functioning of enzymes, cobalt plays an important role in ensuring appetite, growth and vigour. The signs of a shortfall of cobalt can include, for example, reduced appetite, reduced growth rates, reduced feed efficiency, greater susceptibility to infection, poor calf and lamb vigour at birth and dull coats (MacPherson, et al. 1987, Ferguson, Mitchell and MacPherson 1989). Cobalt shortfall is most common in lambs and is often reported as a “growth check” in lambs at weaning. This is most common where they are on pasture in late summer/autumn, especially where cobalt is not present in adequate quantities in the soils or diet. Young calves and lambs that are not yet ruminating are entirely reliant on vitamin B12 stores built up in their liver before birth, and its continued provision via colostrum and milk. Ensuring the mother has an adequate supply of cobalt throughout pregnancy ensures that her offspring’s liver vitamin B12 stores are adequate at birth, and that she can provide enough vitamin B12 in her colostrum and milk to maintain the cobalt status of her young (Gruner, et al. 2004).
Clinical cobalt deficiency, often known as pine, is rare, but clinical signs are watery eye discharge, lethargy, poor quality wool with an open fleece, anaemia, wasting and poor body condition despite adequate nutrition. There may be tear staining of the cheeks, and pale mucous membranes.
Copper
Copper plays an essential role in the activity of many enzymes and processes in an animal’s body. The enzymes that copper is involved in have catalytic, physiological and structural roles in the body. Adequate copper status is crucial for fertility, growth, bone development, immunity and hair/wool pigmentation in cattle and sheep; as well as wool production in sheep. Signs of copper shortfall include poor growth, reduced fertility and increased susceptibility to disease. In more extreme cases of copper deficiency, signs include loss of pigmentation, nervous disorders (swayback), limb and bone abnormalities (Suttle and Angus 1976), osteoporosis, and fractures (Suttle, Angus and Nisbet, et al. 1972, Mills, Dalgarno and Wenham 1976, Suttle and Angus 1978). Copper shortfall or deficiency occurs when the availability of copper in the diet is below animal requirement. This can be a result of a primary deficiency or secondary deficiency.
Primary deficiency
Primary copper deficiency is caused by consumption of a diet that has a copper concentration below animal requirement. This is much less common than secondary deficiency.
Secondary deficiency
Secondary copper deficiency is caused by a reduction in biological availability of copper caused by other dietary constituents known as antagonists, for example molybdenum, sulphur and iron. Antagonists interfere with the absorption of copper from the digestive tract and its metabolism once absorbed; leading to the animal exhibiting signs of a copper shortfall. Molybdenum and sulphur act together to restrict the availability of copper to the animal, reacting together in the rumen to form thiomolybdates. Thiomolybdates react with copper to form insoluble copper thiomolybdates, which cannot be absorbed by the small intestine, resulting in a loss of copper in the faeces. Thiomolybdates can be absorbed in the rumen into the bloodstream and may interfere with copper uptake, and reduce storage in the liver (Mason, et al. 1988, Suttle, Brebner, et al. 1992).
Higher sulphur ingestion enhances the influence of Mo on restricting copper availability (Suttle and McLauchlan 1976, Suttle 1974, Suttle 1975). Pastures can often vary in the amount of Mo present, having been shown to be impacted by season, application of Mo-containing fertilisers, soil type (especially peaty soils), meaning that careful attention should be given to monitoring Mo content of pasture for potential copper availability impacts. High iron intake has also been shown to interfere with the absorption of copper in sheep and cattle.
N.B There are genetic differences in copper requirement between sheep breeds, therefore copper should not be supplemented to animals that are susceptible to copper toxicity.
Iodine
Iodine is essential to the synthesis of the hormones produced by the thyroid gland. The thyroid gland is important to regulate metabolism, thermoregulation and reproduction, and can help to promote weight gain (Scott, Maunsell and Willis 1983). An iodine shortfall often presents as weak, dopey calves, and lambs that are slow to suckle after birth. Inadequate thyroid hormone production and release negatively impacts upon growth rate, milk yields, lambing percentage, and can cause lamb/calf death at birth and several reproductive issues (Davis and Barry 1983). Ensuring adequate iodine status in the mother also ensures that thyroid hormone levels in new-borns are sufficient, allowing them to better withstand cold and wet weather just after lambing (Sargison, West and Clark 1997, Canzanelli, Guild and Rapport 1975). In extreme cases of iodine deficiency, goitre swelling in the neck, caused by an enlarged thyroid gland, can be observed.
Primary deficiency
Deficiencies or imbalances of iodine are often multifactorial, and include dietary, management and environmental factors. Primary iodine deficiency is caused by consumption of a diet that has an iodine concentration below animal requirement.
Secondary deficiency
Secondary deficiency is caused by the presence of other ingested molecules which interfere with iodine uptake and utilisation. The feeding of rape, kale, root (e.g. turnip and swede), and other brassica crops, as well as white clovers, can inhibit the absorption of iodine into the body. Brassica crops contain goitrogens, either thiocyanate or thiouracil compounds. Thiocyanates interfere with the uptake of iodine into the thyroid and can increase iodine requirement up to 4-fold. Adequate selenium status is important to the functioning of the thyroid gland and iodine utilisation, therefore low selenium levels can result in an impaired thyroid function which presents as an apparent iodine deficiency.
Selenium
Selenium is an essential component of many enzymes that play an essential role as antioxidants. They therefore form an important component of animal immunity, thyroid hormone regulation and are involved in the regulation of growth and fertility. Selenium promotes growth by optimising the function of muscles and vital organs, and systems such as the liver, nervous system, immune system and reproductive tracts. Inadequate selenium status often presents as increased susceptibility to disease, poor growth rates, reduced fertility (male and female), increased number of abortions soon after conception, poor lamb/calf vigour, increased number of retained cleansings and impaired wool production. In extreme cases of selenium deficiency, it can cause reduced milk fat yield and white muscle disease.
Primary deficiency
Primary selenium deficiency is caused by consumption of a diet that has a selenium concentration below animal requirement. This is the most common form of selenium deficiency. Forages in many geographical locations do not contain adequate selenium to meet animal requirement.
Secondary deficiency
A secondary deficiency occurs where adequate selenium is provided to the animal, but this selenium is prevented from being utilised fully by other factors. Selenium bioavailability is reduced by high dietary sulphur and the presence of specific toxins (cyanogenetic glycosides) in certain legumes e.g. white clovers (Gutzwiller 1993).
Selenium and Vitamin E
Selenium and vitamin E should not be considered in isolation as they work mutually in a variety of metabolic processes. The effects of selenium and vitamin E are complementary and in some instances the excess of one can spare deficiency of the other. The period around birth is a crucial one for selenium and vitamin E status of the mother and offspring in both cattle and sheep. Vitamin E is poorly transported across the placenta and offspring rely on colostrum for their early supply. The mother, therefore, secretes large quantities of selenium and vitamin E in her milk, which can cause a dramatic reduction in her own blood levels around parturition. The mother would then require further supplementation to maintain her own levels and ensure that her young continue to receive an adequate supply via milk until they can build up their own supplies from forage post-weaning.
Manganese
Manganese is important for the correct functioning of a wide range of enzymes in cattle and sheep. Many of these are involved in the synthesis of bone, teeth and hormones and the production of glucose from dietary precursors. Sheep diets usually contain adequate levels of manganese, however dairy and beef diets, especially those with a high maize content, can be deficient. Deficiency is uncommon in the UK, but signs include poor growth, skeletal deformities in new-borns and reproductive abnormalities, including delayed or irregular oestrus and poor conception rates.
Zinc
Zinc is involved in many biochemical processes, primarily enzymes and a deficiency affects a wide range of body functions. These enzymes are essential to nucleic acid, protein and carbohydrate metabolism, and play a role in the expression of DNA and immune system functioning. The body has no significant long-term stores of zinc, so continual supply is required. Zinc deficiency, which is uncommon in ruminants in the UK, can lead to excessive salivation, deterioration of hair or wool texture, stiff joints and thick, scaly, cracked skin, accompanied or preceded by poor growth. Reproductive function can also be impaired, especially in male animals. Dietary factors, for example increased calcium intake, can affect the absorption of zinc by the gut.
Vitamin A
Vitamin A (retinol) has many functions. It is important for the effectiveness of the immune system, enabling the correct functioning of almost all immune cells, thus helping to provide protection against viral, bacterial and protozoan infections. Vitamin A is associated with maintenance of protective membranes of the respiratory and digestive tracts. Deficiencies of vitamin A often cause damage to these membranes, allowing pathogens to invade more easily. Vitamin A is also important for optimal eye and kidney functioning, as well as the normal development of bones, teeth and nerve tissue.
Vitamin A does not occur as such in plant material; it is synthesised in the animal from its precursors found in various forms, particularly in fresh grass (carotenes and carotenoids). These precursors can be quickly degraded. For example, ensiling can cause some of these precursors to become less available to the animal and this can be a problem when animals are housed for long periods. Cereal grains (except from yellow maize) have poor vitamin A precursor content.
Vitamin D3
Vitamin D is crucial to the normal development of the skeleton, interacting closely with calcium and phosphorous, in doing so, to promote their incorporation into bone. Vitamin D stimulates transport of calcium and phosphorous at three sites: the intestine, kidneys and bones. Vitamin D plays a crucial role in many aspects of calcium uptake and retention throughout the body (including the intestines and kidneys).
Vitamin E
Vitamin E, like selenium, has a role in the prevention of oxidative stress produced by normal cellular mechanisms during critical stages of an animal’s development and production. Naturally grazed forage diets of ruminants are typically rich in vitamin E. However, the vitamin E concentration tends to fall when the forage is conserved and is highly dependent on the method of conservation. For example, drying to make hay can lead to up to an 80% loss of vitamin E, but ensiling and rapid dehydration can retain most of the vitamin E content (Hidiroglou, et al. 1992).
As detailed above, Vitamin E and selenium should not be considered in isolation as they work mutually in a variety of metabolic processes and their effects are complementary.
Biotin
Biotin is an essential coenzyme in carbohydrate, fat and protein metabolism. It is involved in the maintenance of normal blood glucose levels through its involvement in glucose production, synthesis of long-chain fatty acids and amino acid breakdown. Biotin is also essential in the production of volatile fatty acids (VFAs) by bacteria in the rumen and is required for the synthesis of keratin, an essential component of the claw horn.
Calcium
Calcium is a key component of bone, and is required for the proper functioning of nerves, muscle contraction, regulation of enzymes involved in metabolism, the release of hormones, curd formation in milk and blood clotting. Regulation of calcium levels requires an adequate supply of vitamin D.
Milk fever (parturient paresis) is the most serious disorder of calcium deficiency. It occurs when the demand for calcium in the mother is increased, usually in the time around parturition, causing blood levels of calcium to deplete at a faster rate than calcium can be replenished from body reserves. Sub-clinical milk fever is harder to detect than clinical milk fever and has symptoms which may not show for extended periods. It is estimated to affect up to 54 % of dairy cows, who have had one or more pregnancies after calving, as opposed to 25 % of heifers (Reinhardt, et al. 2011) and is much harder to detect than clinical milk fever. Although cost per individual case is lower, it is estimated that overall herd incidence of sub-clinical milk fever can cost around four times more than clinical milk fever incidence (Oetzel 2012).
Signs of sub clinical milk fever may include low temperature and cold extremities, blood calcium levels below “normal” ≤2.00 mmol/L, poor appetite and unsteady/weak/nervous/excited behaviour. Without intervention, low calcium levels and milk fever can lead to a cascade of productivity consequences with negative impacts, which ultimately reduce dry matter intake, increase metabolic disease incidence and decrease milk yield (Oetzel 2012). Other consequences can include:
- Reduced immune function
- Reduced reproductive performance
- Generalised poor health and productivity
When symptoms of sub clinical milk fever occur, immediate supplementation of calcium is needed to stabilise blood levels and help the animal through the transition period, until it can replenish calcium using its own skeletal stores.
Magnesium
Magnesium is involved in a wide variety of processes in sheep and cattle, acting as a catalyst to hundreds of biochemical reactions. Examples of these include regulation of the interface between the nervous system and muscles, conversion of sugars and fatty acids to energy, regulation of fluid content in the cells and body and regulation of the heart.
Clinical signs of magnesium deficiency (grass tetany/staggers/hypomagnesaemia) can occur very quickly as the body has little stores of magnesium compared with its requirement and so is therefore reliant on dietary intake. Preventative supplementation is normally recommended as there are few warning signs before clinical symptoms occur and a rapid deterioration and death is common. Deficiency is most common in lactating cattle at grass (especially in negative energy balance) as magnesium content of grass, especially rapidly growing grass found in spring and autumn, can be very low and output in milk high.
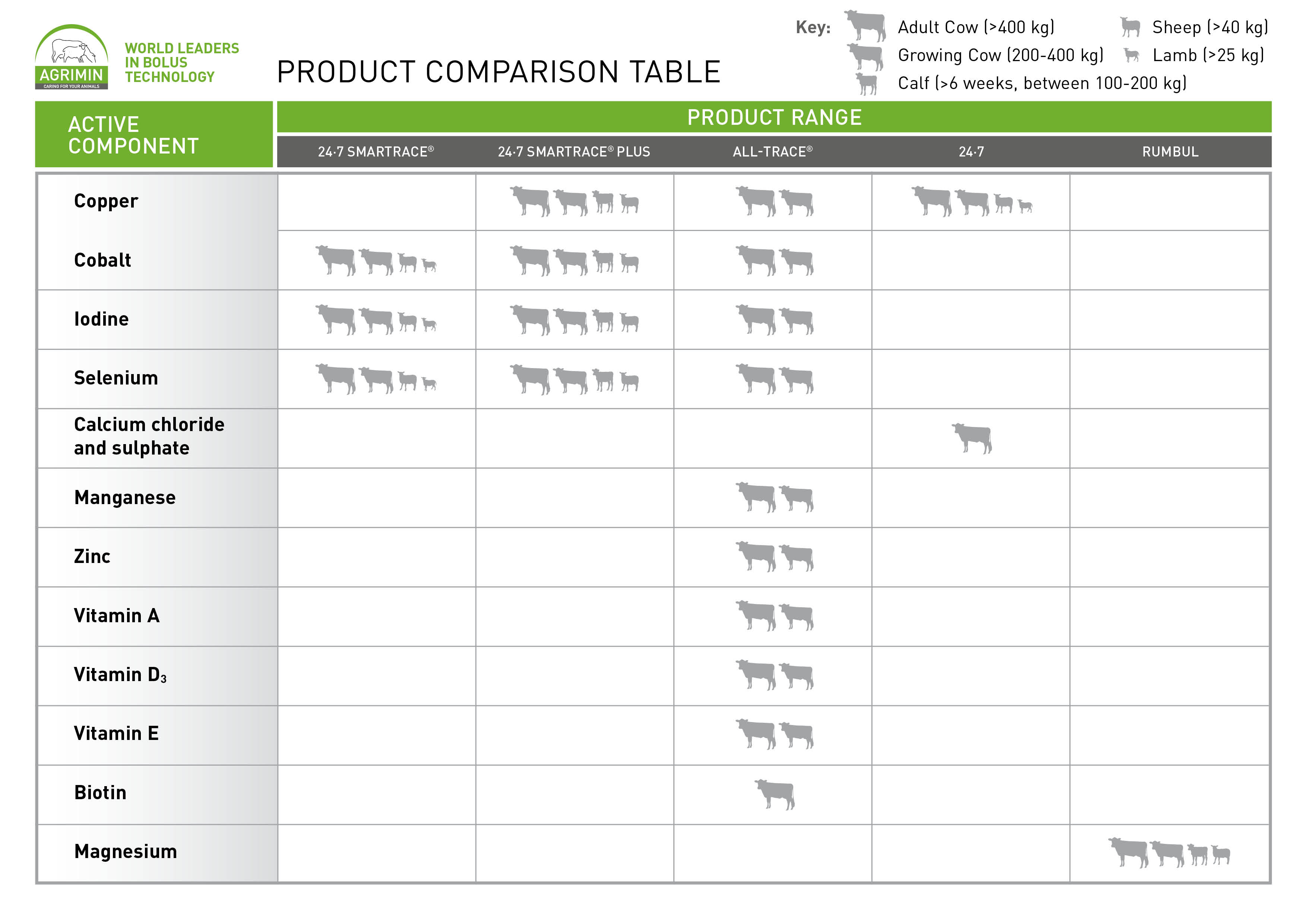
The following table shows which Agrimin products contain which active component and which animals they are suitable for:
Bibliography
Canzanelli, A., R. Guild, and D. Rapport. 1975. “Further observations on the effect of thyroglobulin and thyronin on O2 consumption of tissues in vitro.” Endocrinology 1080-1088.
Davis, G. H., and T. N. Barry. 1983. “Responses to iodine supplementation in high fecundity Booroola Merino x Romney ewes.” Proceedings of the Nutrition Society of New Zealand 8: 110.
Ferguson, E. G., G. B. Mitchell, and A. MacPherson. 1989. “Cobalt deficiency and Ostertagia circumcincta infection in lambs.” Veterinary Record 12 (1): 20. doi:10.1136/vr.124.1.20.
Grace, Neville D, Scott O Knowles, and Andrew R Sykes. 2010. Managine Mineral Deficiences in Grazing Livestock. Hamiltin: New Zealand Society of Animal Production.
Gruner, T. M., J. R. Sedcole, J. M. Furlong, N. D. Grace, S. D. Williams, G. Sinclair, and A. R. Sykes. 2004. “Changes in serum concentrations of methylmalonic acid and vitamin B12 on cobalt-supplemented ewes and their lambs on two cobalt-deficient properties.” New Zealand Veterinary Journal 52: 117-128. doi:10.1080/00480169.2004.36416 .
Gutzwiller, A. 1993. “The effect of a diet containing cyanogenetic glycosides on the selenium status and thyroid function of sheep.” Animal Production 57: 415-419.
Herdt, T.H. n.d. Nutritional Requirements of Dairy Cattle. Accessed 2 21, 2019. http://www.merckvetmanual.com/mvm/management_and_nutrition/nutrition_dairy_cattle/nutritional_requirements_of_dairy_cattle.html.
Hidiroglou, N, N. Cave, As Atwal, Er Farnworth, and Lr Mcdowell. 1992. “Comparative vitamin E requirements and metabolism in livestock.” Annales de Recherches Vétérinaires 23 (4): 337-359.
MacPherson, A, D Gray, G. B. Mitchell, and C. N. Taylor. 1987. “Ostertagia infection and neutrophil function in cobalt-deficient and cobalt-supplemented cattle.” British Veterinary Journal 143 (4): 348-353. doi:10.1016/0007-1935(87)90069-8 .
Mason, J., M. Lamand, J. C. Tressol, and G. Mulryan. 1988. “Studies of the changes in systemic copper metabolism and excretion produced by the intravenous administration of trithiomolybdate in sheep.” British Journal of Nutrition 59 (02): 289-300. doi:10.1079/bjn19880036.
McGuffey, R.K. 2017. “A 100-Year Review: Metabolic modifiers in dairy cattle nutrition.” Journal of Dairy Science 100 (12): 10113-10142. Accessed 2 21, 2019. https://sciencedirect.com/science/article/pii/s0022030217310457.
Mills, C. F., A. C. Dalgarno, and G. Wenham. 1976. “Biochemical and pathological changes in tissues of Friesian cattle during he experimental induction of copper deficiency.” British Journal of Nutrition 35: 309-335.
Oetzel, G. R. 2012. “An Update on Hypocalcemia on Dairy Farms.” Proceedings of the Four-State Dairy Nutrition & Management Conference. Dubuque, Iowa. 80-85.
Oetzel, G. R. 2011. “Non-Infectious Diseases: Milk Fever.” In Encyclopedia of Dairy Sciences, by J. W. Fuquay, 239-245. Academic Press.
Royal DSM. 2014. Vitamin Nutrition Compendium - Ruminants. 12 November. Accessed March 19, 2019. https://www.dsm.com/markets/anh/en_US/Compendium/ruminants.html.
Sargison, N. D., D. M. West, and R. G. Clark. 1997. “An investigation of the possible effects of subclinical iodine deficiency on ewe fertility and perinatal lamb mortality.” New Zealand Veterinary Journal 45: 208-211.
Scott, D., L. A. Maunsell, and B. H. Willis. 1983. “Lack of weight gain of sheep following iodine supplementation in inland regions of the South Island.” New Zealand Veterinary Journal 31: 120-122.
Skerman, K. D., M. W. O'Halloran, and B. L. Munday. 1961. “The effect of cobalt bullets on milk production of dairy cattle.” Australian Veterinary Journal 37 (5): 181-184. doi:10.1111/j.1751-0813.1961.tb07851.x .
Stathan, M., and A. C. Bray. 1975. “Congenital Goitre in Sheep in Southern Tasmania.” Australian Journal of Agricultural Research 24: 751-768. doi:10.1071/ar9750751 .
Suttle, N. F. 1974. “Recent studies of the copper-molybdenum antagonism.” Proceedings of the Nutrition Society. 299-305. doi:10.1079/PNS19740053.
Suttle, N. F. 1975. “The role of organic copper in the copper-molybdenum-sulphur interrelationship in ruminant nutrition.” British Journal of Nutrtition 34 (3): 411-420. doi:10.1017/S0007114575000475.
Suttle, N. F., and K. W. Angus. 1978. “Effects of experimental copper deficiency on the skeleton of the calf.” Journal of Comparative Pathology 88: 137-147.
Suttle, N. F., and K. W. Angus. 1976. “Experimental copper deficiency in the calf.” Journal of Comparative Pathology 86: 595-608. doi:10.1016/0021-9975(76)90068-2.
Suttle, N. F., and M. McLauchlan. 1976. “Predicting the effects of dietary Mo and sulphur on the availability of copper to ruminants.” Proceedings of the Nutrition Society. 22.
Suttle, N. F., J. Brebner, J. Small, and K. McLean. 1992. “Inhibition of ovine erythrocyte superoxide dismutase (ESOD: EC .14.1.1) activity in vivo by parenteral ammonium tetrathiomolybdate.” Proceedings of the Nutrition Society. 145A. Suttle, N. F., K. W. Angus, D. I. Nisbet, and A. C. Field. 1972. “Osteoporosis in copper-depleted lambs.” Journal of Comparative Pathology 82: 93-97. doi:10.1016/0021-9975(72)90031-X.
Underwood, E. J., and N. F. Suttle. 1999. The Mineral Nutrition of Livestock. 3rd. Wallingford, Oxon: CABI Publishing.





